Advancing Biofabrication at 3D BioFibR with the FibrCatR 2.0
Working with Enginuity as a local partner made all the difference. Having their team close by meant fast, hands-on collaboration, and being able to share real materials for rapid prototyping was invaluable. Enginuity’s accessibility and expertise were a big part of our project’s success.
Dana Bhattacharyya
Director of Operations, 3D BioFibR
About the Client
3D BioFibR
With a diverse, multidisciplinary team, 3D BioFibR maintains a partnership-driven model, co-developing with their clients but focusing on scalable, manufacturing-centric solutions rather than end-market positioning or regulatory strategies.
Understanding the Problem
The Challenge
After launching the original FibrCatR 1.0 to develop collagen fibre matrices, 3D BioFibR quickly encountered several technical and operational bottlenecks:
- Limited Motion and Dimensionality: The motion path and hardware of FibrCatR 1.0 restricted the ability to produce complex, three-dimensional, crosshatched scaffolds required for many of their target markets’ applications.
- Material Efficiency Issues: The original solution dispensing relied on syringe pumps with minimum usable volumes (about 10mL), but most prototype runs required just 1–2mL. Since source proteins are expensive, reducing waste was critical.
- Inconsistencies and Bugs: The rapid prototyping techniques utilised to get the first production identified deficiencies in structure, repeatability and reliability that need to be addressed
- Cleanroom Compliance: As 3D BioFibR pursued ISO 13485 certification, all equipment requires risk assessments and cleanroom compatibility.
- Modularity and Upgradability: The initial design restricted further adaptation for new formats or future production needs.
Automation and Mechanical Team
Enginuity’s Mechanical and Automation Teams standing proudly after Factory Acceptance Test (FAT)

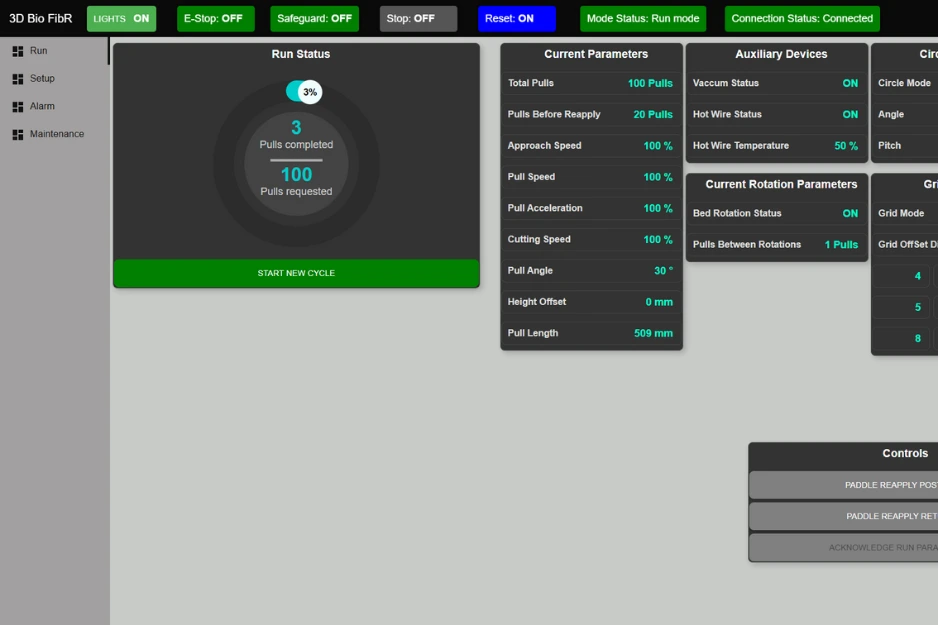
Human-Machine Interface (HMI)
Customized HMI for complete control and monitoring of the system
Robot Integration
6 Axis Epson robot and actuator/paddle in mid-pull

Solving the Challenge
The Solution
Recognizing these bottlenecks, 3D BioFibR collaborated closely with both Enginuity mechanical design and automation teams to reimagine the system for FibrCatR 2.0. Key solution elements included:
- Integration of Robotic Arms: An off-the-shelf 6-axis robotic arm replaced the T-rails, allowing for highly precise, flexible, and reproducible motion paths. The robotic arms came with robust, well-tested software, safety-sensing to prevent collisions, and the capacity for various fiber-pulling motions (A-frame, perpendicular, adjustable speed).
- Process and Product Tunability: System redesign enabled easy shifts from small to large scaffolds, adjustment of paddle motions, and creation of specialized 3D architectures.
- Reduced Material Waste: The upgraded solution dispensing accommodated much smaller batch volumes, minimizing expensive raw protein wastage.
- Cleanroom Compatibility: Components of FibrCatR 2.0 underwent risk assessment and machine design adjustments to ensure full compliance with ISO 13485 cleanroom standards aligning with future production strategy.
- Improved Modularity: The new architecture was designed for rapid adaptation, allowing the system to be reconfigured for alternate product formats, such as yarns or different well-plate sizes, to support diverse customer needs.
Summarizing the Outcome
The Conclusion
Pending final site acceptance testing (SAT), which all parties expect to pass without issue, the anticipated results include:
- 10-fold (10X) Increase in Throughput: Compared to FibrCatR 1.0, the new version is projected to deliver a dramatic boost in scaffold production capacity.
- Highly Consistent, Higher-Quality Products: Automation and precision engineering reduce variation, leading to improved bio-compatibility and reproducibility of final products.
- Minimized Human Intervention: Enhanced automation reduces reliance on manual adjustments, reduces human error while improving operator safety.
- Seamless Integration: The new system supports easy integration with larger manufacturing or quality control systems, representing a scalable solution ready for regulated environments.
Key Takeaways
Local Collaboration Drives Innovation
Tangible Prototype
Future-Proofs Manufacturing
The flexible, reconfigurable system architecture ensures 3D BioFibR can quickly adapt to new business opportunities or scientific advances.




